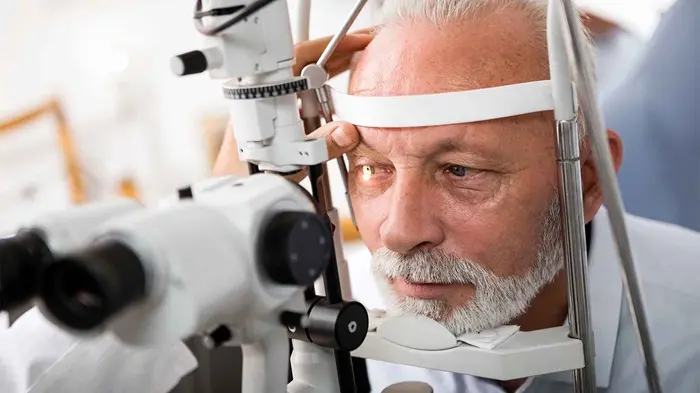
Alcon has completed the acquisition of Belkin Vision and its DSLT laser device designed for treating glaucoma. The deal involved an initial payment of $81 million, including $65 million in cash, with potential additional payments of up to $385 million contingent on achieving sales milestones. The technology aims to reduce intraocular pressure without the use of medications or eyedrops, which can otherwise lead to optic nerve damage if untreated.
Belkin’s Eagle device administers 120 pulses of laser energy to the trabecular meshwork near the cornea, aiding in the drainage of excess fluid. Distinguished by its use of eye-tracking technology, the system offers a potentially hands-free approach, setting it apart from previous SLT methods.
Sean Clark, president of Alcon’s global surgical franchise, emphasized the strategic significance of DSLT in advancing SLT as a first-line treatment for glaucoma. The technology, already approved in the EU, UK, and with a 510(k) clearance from the FDA, is slated for commercial release in the U.S. by the end of this year.
In addition to DSLT, Alcon’s glaucoma portfolio includes microstents like the Hydrus and pharmaceutical drops. The company plans to integrate DSLT into its Alcon Vision Suite training platform for healthcare providers, aiming to expand accessibility to this innovative technology.
“We are committed to addressing unmet needs in glaucoma treatment by enhancing access to this transformative technology,” Clark remarked. Previous clinical trials have indicated that SLT approaches are cost-effective for primary treatment of open-angle glaucoma and ocular hypertension, potentially reducing reliance on repeated medications and surgeries while streamlining clinical workflows.
Related topics: